Indonesia Medical Device Market
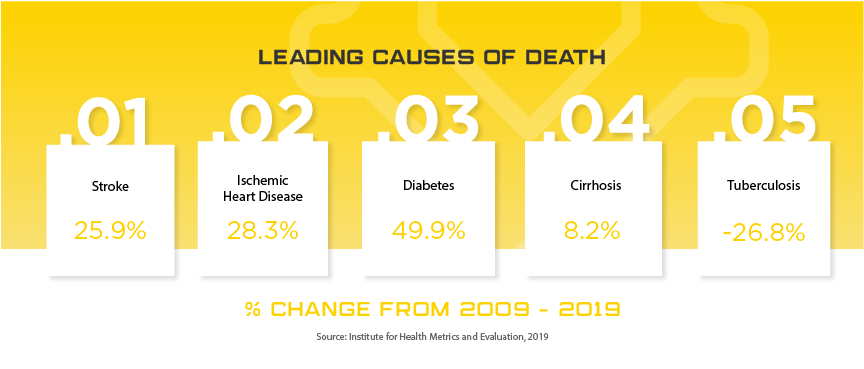
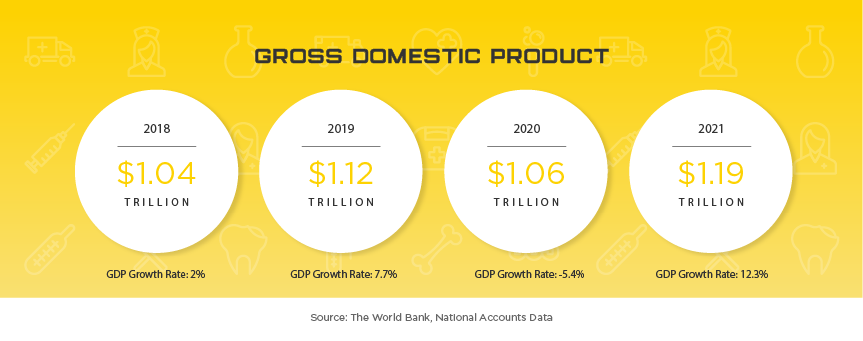
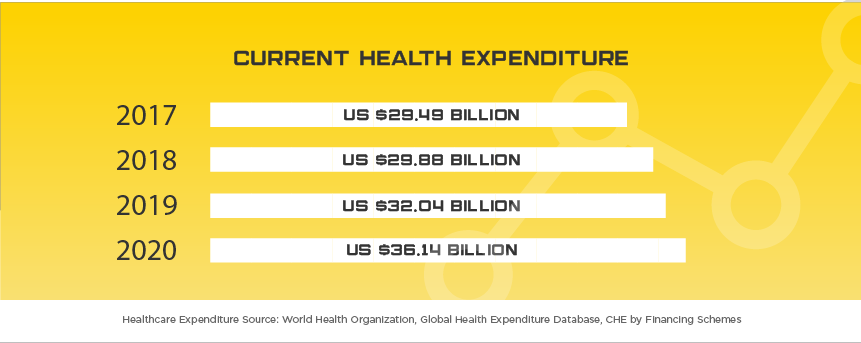
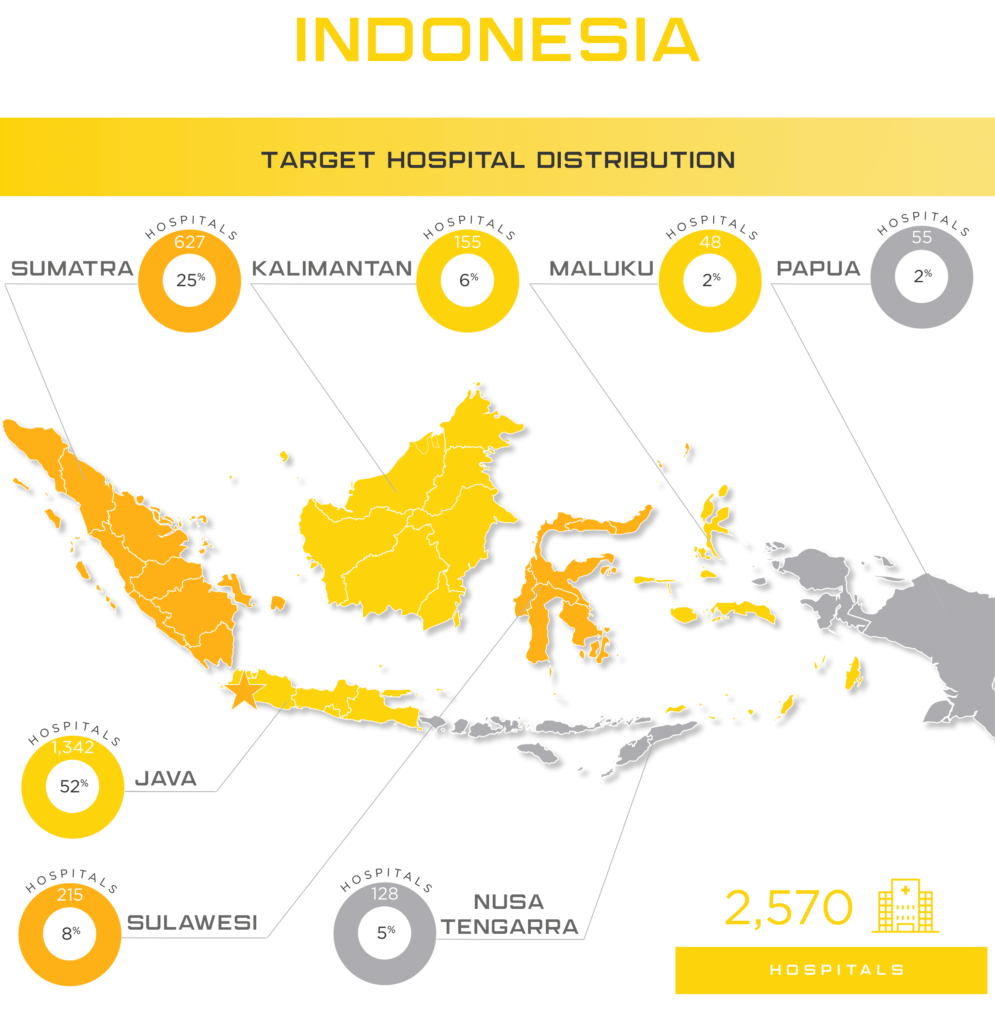
Indonesia is one of the newest Asian markets to present foreign mid and smaller size medical device companies with significant opportunities largely due to their medical device regulatory and importation requirements becoming more transparent in recent years. Indonesia offers medical device manufacturers a lucrative, developing market where foreign brands and market share are more up for grabs than neighboring markets of similar size. The Indonesia Ministry of Health (MoH) oversees the island nation’s medical device industry, which serves 264 million people, with expected yearly growth of nearly 10% to $1.5 billion by 2022. Some of the major players in the market include Siloam Hospitals, Omni Group, Mayapada Group and Sinar Mas Group. As in other developing Asian countries, Indonesia is facing an increase in “Western” health issues such as diabetes where they rank among the top 10 countries afflicted. Given the geographical challenges Indonesia faces, medical device manufacturers that can provide services to people in remote areas will have the opportunity to expand throughout the country as the government invests in expanded, quality healthcare to all of their citizens. When combined with the lack of local manufacturers and expected growth in medical device imports of up to 40% per year, Indonesia is one of the next unexplored frontiers for mid to small size medical device manufacturers around the world.
Grow with us
Asia Actual is available to help navigate the complex medical device registration requirements and regulatory pathway for medical device and IVD distribution in Indonesia. Contact Asia Actual for a free consultation discussing the potential for your medical device or IVD in the Indonesia medical device market.
Important Documents and Links
Indonesia Holidays
- January 1 – New Year’s Day
- January 28 – Chinese New Year
- March 28 – Hari Raya Nyepi
- April 14 – Good Friday
- April 24 – Isra Miraj
- May 1 – Labour Day
- May 11 – Waisak Day
- May 25 – Ascension Day
- June 1 – Pancasila Day
- June 23 – Cuti Bersama
- June 25 – Hari Raya Puasa
- June 26 – Hari Raya Puasa Holiday
- June 27 – Cuti Bersama
- June 28 – Cuti Bersama
- August 17 – Independence Day
- September 1 – Idul Adha
- September 21 – Muharram
- December 1 – Maulidur Rasul
- December 25 – Christmas Day
- December 26 – Cuti Bersama
Indonesia Regulatory Support

Ilham Hidayattulah
Local Office

Contact Us
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: Indonesia@asiaactual.com
Latest Market Updates
 Indonesia to Require Post-Market In-Country Testing of Medical DevicesOctober 11, 2023 - 10:04 am
Indonesia to Require Post-Market In-Country Testing of Medical DevicesOctober 11, 2023 - 10:04 am Indonesia e-Catalogue Implementation Review, December 2022December 30, 2022 - 11:45 am
Indonesia e-Catalogue Implementation Review, December 2022December 30, 2022 - 11:45 am Good Distribution Practice Requirements in IndonesiaOctober 28, 2022 - 2:47 pm
Good Distribution Practice Requirements in IndonesiaOctober 28, 2022 - 2:47 pm How New EU MDR Requirements Will Affect Registrations in AsiaAugust 12, 2022 - 2:07 pm
How New EU MDR Requirements Will Affect Registrations in AsiaAugust 12, 2022 - 2:07 pm Indonesia Issues New Guidance for Freezing Medical Devices on eCatalogueAugust 5, 2022 - 9:30 am
Indonesia Issues New Guidance for Freezing Medical Devices on eCatalogueAugust 5, 2022 - 9:30 am
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
Asia Actual, LLC
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
Asia Headquarters
116 Changi Road, #04-05