Local Office
Unit 1A GV Square Building Casa Milan, Commonwealth Avenue, Greater Lagro, Quezon City, Metro Manila, The Philippines
+63 2 616-3150
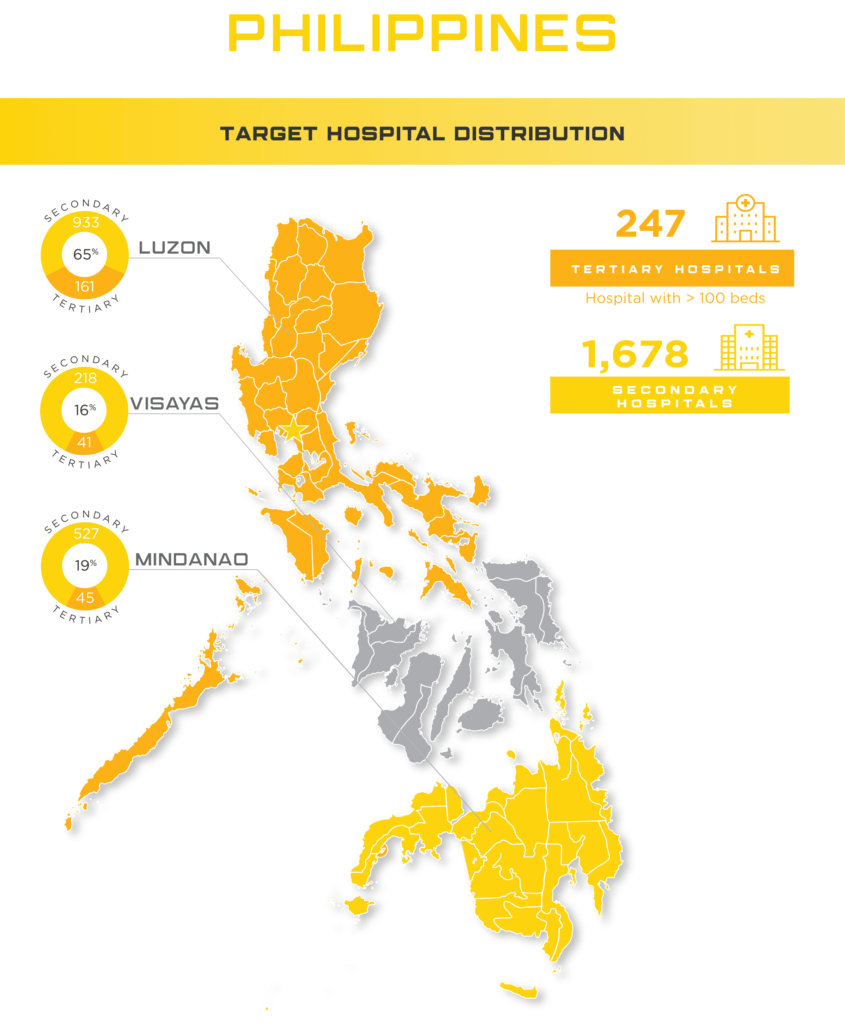
The Philippines medical device market, overseen by the Philippines FDA continues to remain advantageous to foreign medical device manufacturers. In order to provide the full spectrum of products the island nation needs, The Philippines healthcare industry heavily relies on foreign medical device and IVD manufacturer imports. And as a developing market, demand for medical device can be attributed to a steady economic growth of 6% and continued hospital expansion. Currently, there are 4 major healthcare providers:
The government alongside the Philippines Department of Health (DOH), has made healthcare a priority. 94% of their citizens through their social health insurance program, called Philippine Health Insurance (PhilHealth). Given the Philippine’s stage of development, combined with a strong desire for universal coverage, the Philippines medical device market is particularly price sensitive and competition can be fierce among global manufacturers vying for the Philippines current, and future, healthcare market. High potential growth areas include: consumables, x-ray and radiation equipment, breathing appliances, linear accelerators, CT scanners, MRI equipment, ultrasound equipment, biological rapid test kits and resources for treating hypertension and diabetes/kidney disease.
Asia Actual is available to help navigate the complex medical device registration requirements and regulatory pathway for medical device and IVD distribution in the Philippines.
Contact Asia Actual for a free consultation discussing the potential for your medical device or IVD in the Philippines market.


Unit 1A GV Square Building Casa Milan, Commonwealth Avenue, Greater Lagro, Quezon City, Metro Manila, The Philippines
+63 2 616-3150
US: +1 512 898-9222
SG: +65 8800-3197
EMAIL: philippines@asiaactual.com
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
116 Changi Road, #04-05
