Hong Kong Medical Device Market
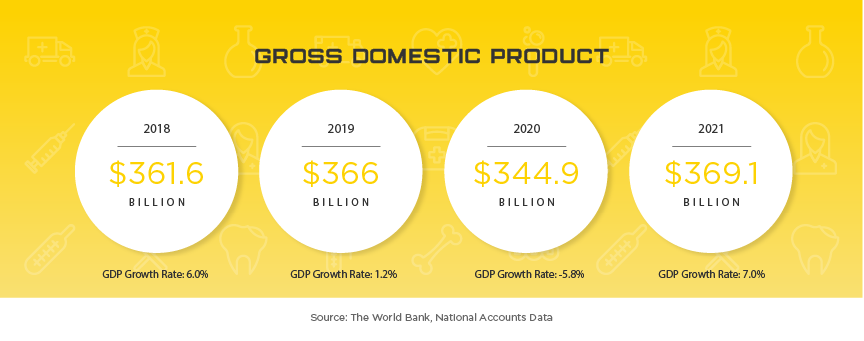
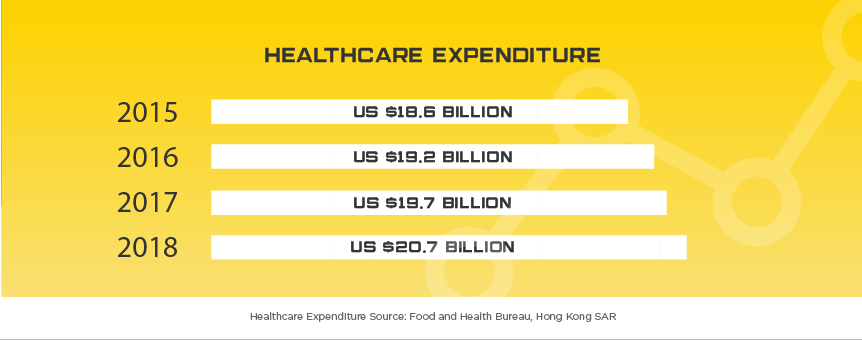
Hong Kong’s affluent and sophisticated medical device industry continues to offer device manufacturers great opportunities. Hong Kong’s long history of wealth and foreign presence has led to decades of industry leading medical service in Asia. Given Hong Kong’s limited space, but high population, the city heavily relies on medical device imports, reaching US$1.86 billion in 2016, 10% over 2015. Like many of their neighboring countries, a lot of this growth is attributed to their aging population with citizens aged 65 or older expected to more than double by 2041, growing from 1.2 million to 2.6 million. While US companies maintain the largest share of any country when it comes to medical equipment imports, total market share has slipped from near 20% to 19% in 2016.
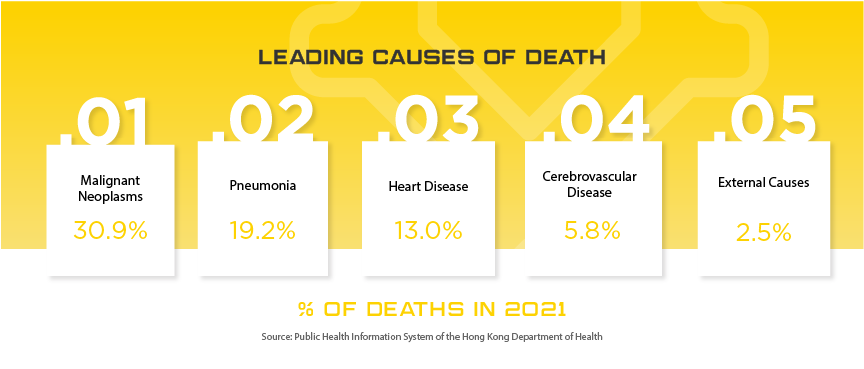
Hong Kong society has been stable, with growing economic development after the national security law was implemented in the city three years ago. With Hong Kong’s unique strengths and the staunch support of the Chinese central government, the overall social stability, economic development and free and convenient business environment have continued to grow. Manufacturers can still consider Hong Kong as a crucial entrance point to China, especially when it comes to cutting edge technology and manufacturers looking for branding opportunities. Specific industries showing strong potential for growth in Hong Kong: cancer treatment, pneumonia care, cardiovascular prevention and treatment, and cosmetic dentistry and oral health.
GROW WITH US
Asia Actual is available to help navigate the complex medical device registration requirements and regulatory pathway for medical device and IVD distribution in Hong Kong.
Contact Asia Actual for a free consultation discussing the potential for your medical device or IVD in this lucrative market.
Important Documents and Links
Hong Kong Regulatory Support
Hong Kong Sales Support

Eric Leung
Contact Us
Address: 17/F Chung Pont Commercial Building
300 Hennessy Road Wan Chai, Hong Kong
US: +1 512 898-9222 SG: +65 8800-3197
EMAIL: HongKong@asiaactual.com
Latest Market Updates
 Hong Kong MDD Adds Singapore as Reference CountryApril 4, 2024 - 8:38 am
Hong Kong MDD Adds Singapore as Reference CountryApril 4, 2024 - 8:38 am Hong Kong MDD Adds China and Korea to List of Reference CountriesJanuary 29, 2024 - 11:36 am
Hong Kong MDD Adds China and Korea to List of Reference CountriesJanuary 29, 2024 - 11:36 am Hong Kong MDD Issues New Technical Reference Document for AI-MDJanuary 22, 2024 - 3:01 pm
Hong Kong MDD Issues New Technical Reference Document for AI-MDJanuary 22, 2024 - 3:01 pm Hong Kong MDD Issues Technical Reference for SaMD and SiMDJanuary 17, 2024 - 4:22 pm
Hong Kong MDD Issues Technical Reference for SaMD and SiMDJanuary 17, 2024 - 4:22 pm MDD Issues Guidance on Making Changes to Medical Device Licenses in Hong KongDecember 7, 2023 - 2:49 pm
MDD Issues Guidance on Making Changes to Medical Device Licenses in Hong KongDecember 7, 2023 - 2:49 pm
実際の亞洲
เอเชีย แอคชวล
एशिया वास्तविक
실제 아시아
Asia Actual, LLC
515 Congress Avenue, Suite 2100
Austin, TX 78701
+1 512 898-9222
Contact Us
Privacy Policy
Asia Headquarters
116 Changi Road, #04-05